Regulation of Mesenchymal Stem Cell Multipotentiality: Interplay of Matrix Stiffness and Matrix Ligand Presentation
Mesenchymal stem cells from a variety of sources have shown enormous promise in preclinical studies to facilitate bone and cartilage regeneration. Despite this promise, the therapeutic potential of these cells relies upon identifying the necessary cues governing their self-renewal and multi-lineage maintenance. A myriad of factors are known to influence stem cell fate, including soluble and insoluble factors, cell-extracellular matrix (ECM) interactions, and mechanical forces. We hypothesize that matrix compliance and composition modulate the maintenance of mesenchymal stem cells (MSCs) in an undifferentiated state in vitro. The long-term goal is to use these cells in cartilage regeneration strategies.
Reprogramming of Mesenchymal Stem Cells for Cartilage Tissue Engineering
Clinical application of cartilage tissue engineering strategies using adult stem cells requires constant stabilization of cell behavior, which, to date, investigators have had variable success with through ubiquitous exposure of the cells to growth factors. However, stem cells go through several stages of maturation as they become cartilage-producing cells (chondrocytes) and at each stage, the response of the cell to growth factors may be different. The objective of this proposal is to use gene transfer to concurrently manipulate pivotal intracellular pathways that regulate cartilage cell maturation and matrix remodeling as a means to direct adult stem cell maturation and matrix accumulation in vitro for development of clinically viable cartilage repair strategies.
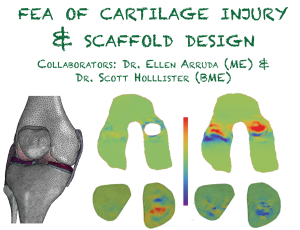
Evaluation of a Novel Bioresorbable Polymer Scaffold for the Treatment of Traumatic Cartilage Injuries
Articular cartilage injury is a major clinical problem and current surgical and tissue engineering strategies do not fully regenerate injured cartilage. Most cartilage defects inevitably lead to osteoarthritis, a painful and debilitating disease. Three dimensional polymer printing enables investigators to rapidly produce objects of complex structure in a very short time. With this technology, it would be possible to produce a scaffold that fits the geometry of a defect based on arthroscopic or magnetic resonance images of the injured area. The objective of this project is to evaluate a scaffold with a novel design whose mechanical properties will be manipulated throughout the height of the scaffold using 3D printing. We will construct a finite element model based on the specific geometries of human bones and cartilage defects using patient scans and assess the stresses produced in the tissue due to interaction with the scaffold. Several parameters of the scaffolds geometry, such as the porosity, will be varied to control the mechanical properties and prevent the formation of loading environments that have been shown to be detrimental to the health of the remaining cartilage tissue. These results will be confirmed in an in vitro model of cartilage loading and the effect on cartilage biochemistry and cell viability will be quantified. Finally, scaffolds with optimized parameters will be manufactured based on the contours of cadaveric knees and the resulting contact mechanics will be compared to that of healthy joints and knees with osteochondral defects. The ultimate goal is to produce a scaffold that will restore the contact mechanics in injured human knees to that of the intact condition, thereby preventing loading conditions that will lead to osteoarthritis.